PGDx elio tissue complete enables you to confidently advise your oncologists and gain new research advantages with our sample-to-answer IVD kit that includes fully automated bioinformatics and dedicated customer support.
PGDx elio™ tissue complete
The first and only FDA-cleared tumor profiling kitted solution
Thank you for your submission.
Product overview
Benefits of the PGDx pan solid tumor CGP test
Our test, your lab
Offer rapid comprehensive, scalable genomic testing and report on actionable findings with confidence with 5 day* turnaround from isolated nucleic acid to variant report.
Accelerate your path to test launch and reimbursement
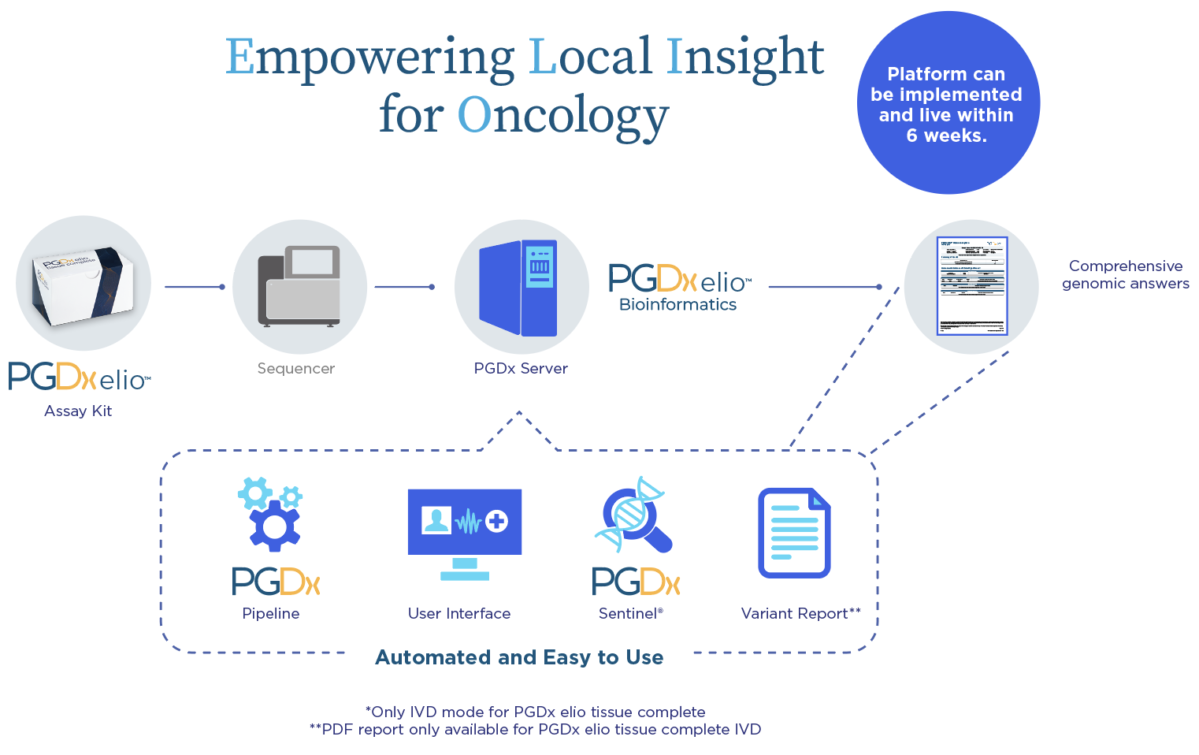
FDA-cleared and CE-marked solution allows for verification, not validation. Professional services available with dedicated implementation project management with custom script development enables seamless integration. Go-live in 6 weeks.
Enable new opportunities
Activate new research opportunities and unlock new potential paths to reimbursement.
Gene panel with TMB and MSI
Solid tumor types
CE-marked
Days from isolated nucleic acid to variant report*
Weeks to go live
People covered for Medicare reimbursement
*Kitted workflow plus pipeline output. Not inclusive of DNA extraction and downstream processing
Market leaders choose PGDx elio™ tissue complete
This test identifies somatic mutations with high accuracy and sensitivity, providing information on single nucleotide variants (SNVs), insertions/deletions (indels), amplifications, translocations, microsatellite instability (MSI) status and tumor mutation burden (TMB) for use by qualified professionals to guide treatment decisions.
Resources
& sample reports
Patent Disclaimer
For In Vitro Diagnostic Use.
Personal Genome Diagnostics Inc. (PGDx) is a subsidiary of Laboratory Corporation of America Holdings, using the brand Labcorp.
©2023 Laboratory Corporation of America® Holdings. All rights reserved.